
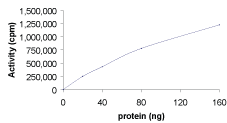
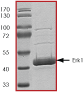
ERK1, Active
Recombinant full-length, tag-free human ERK1 was expressed in E.coli cells and activated by active MEK1 in vitro.
Catalog No. M29-10U
| Catalog No. | Pack Size | Price (USD) | |
|---|---|---|---|
| M29-10U-05 | 5 ug | $226 | |
| M29-10U-10 | 10 ug | $325 | |
| M29-10U-BULK | BULK | Contact Us |
 RAF1 (EE), Active, R01-13G
RAF1 (EE), Active, R01-13G

 Toll Free: 1-866-954-6273
Toll Free: 1-866-954-6273 info@signalchem.com
info@signalchem.com